
Watercooler conversations January 25, 2024
FDA guidance document Use of International Standard ISO 10993-1, "Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process" is probably the most used guidance document for medical devices. It is regularly referred to during biological evaluations and FDA submissions. Many of us carrying out biological evaluations of medical devices can quote sections from it at the drop of a hat.
So, it came as a pretty big surprise when FDA announced in September that the guidance, which had just been revised in 2020, was revised again. The announcement raised questions for many in the industry regarding what changed what FDA's new expectations are regarding submissions or biological evaluations in general.
The biggest and the most welcome change was the inclusion of attachment G, where the long-awaited draft guidance on intact skin contact was finally incorporated into the final document. We should take a moment to celebrate this milestone, an important step forward in recognizing that some devices and some materials are simply so low risk that pre-clinical testing should not be recommended, especially using the animal models that are most often applied. With the inclusion of attachment G, from now on, when a medical device is made from recognized (listed) material(s) and has only intact skin contact, FDA will no longer require submission of test reports. This, of course, does not mean that a risk assessment is not needed to indicate low risk, but at least the debate on why we should not perform another round of irritation and sensitization testing on well-known materials can hopefully be avoided.
As stated in this new revision of the guidance document, “FDA has identified specific materials in the final finished devices that are included in the policy outlined in this attachment when they are in contact with only intact skin surfaces. The materials can include other processing chemicals and additives (e.g., plasticizers, fillers, color additives, cleaning agents, mold release agents). Except for color additives, these chemicals would not need to be disclosed in a marketing submission for devices with this type of tissue contact.”
To note though, attachment G only applies to certain materials with intact skin contact. There are many clauses written where this does not apply. For instance, neonatal skin contact is excluded from this approach due to the neonatal skin’s continued formation toward full protective barrier properties.
Another big change includes the reference to FDA’s accreditation scheme for conformity assessment (ASCA) program in the final guidance document. For the past three years the agency has run a pilot program for ASCA to test out the approach and see if it makes the submissions easier. And now, it is fully integrated as a recommendation for testing in the guidance. The aim of the ASCA program is to streamline a submission to the agency based on FDA reviewers’ enhanced confidence in test methods and results from ASCA-certified testing labs, which means fewer questions or additional information requests on test reports, sample preparation, and conformance to the standards. This, of course, does not mean that everyone will need to queue up at ASCA-certified labs and only use ASCA-certified labs or test methods for their FDA submission. Reports from labs that are not ASCA-certified for the specific tests are still acceptable. However, for those studies, the full reports are required to be submitted (which follows the previously used approach).
Table A.1 in the guidance was also updated to list the correct endpoints that are now covered in the ISO 10993-1:2018, with an X indicating the ones that are present in both documents, and an O indicating those that should be considered in addition when submitting to FDA. There were no other mentionable changes. One thing to point out is that ISO 10993-1:2018 Table A.1 separates out subacute and subchronic toxicity endpoints, whereas FDA guidance has them in the same column. This has sometimes given the impression that the expectation would be to always perform these together, but now a clarification has been added that states that the choice and/or duration of test for these endpoints should be based on the duration of device use (e.g., devices used for more than 14 days should not be assessed using a 14-day test).
Another point worth mentioning is that in the background, where lots of discussion within the ISO 10993-1 draft working group has been going on about the necessity to expand genotoxicity assessment requirement for, for instance, all direct blood-contacting devices (even with limited contact), the FDA guidance 2023 revision still stands on the position that only the blood-contacting devices that are part of an extracorporeal circuit should require this endpoint. This, of course, only applies to limited contact devices, as for prolonged- and long-term direct blood-contacting devices, genotoxicity potential should always be considered and addressed.
Another noticeable change in the main body of the text is related to comparison articles, where testing method requires or recommends the use of a comparison article. Previously, the text noted without ambiguity that for all FDA submissions a U.S.-legally marketed device must be used, and many conversations were had where alternatives from non-U.S. markets were not considered appropriate. In the 2023 revision this has been removed from the main text. Unfortunately, however, this is not yet a point of triumph, as the expectation is still present in a footnote that states that, “For the purposes of this guidance, 'legally marketed devices' are limited to devices marketed in the U.S.”
All in all, the document and guidance provided within did not change much.
Even though the changes made to the guidance were not radical (none of them significant other than the inclusion of attachment G), there are still many aspects that are good to revisit and some existing recommendations from FDA that are important to remember. While there were several great topics to choose from, we settled on Test Article Preparation for Extract Testing (which makes up a large chunk of FDA's requests for additional information), the Guinea Pig Maximization Test, Material Mediated Pyrogen (MMP), and Implantation Testing. Careful consideration of the FDA’s guidance in these areas can make a significant impact to any device submission.
Preparing test articles for extract testing is unique for every medical device, so additional care in the instructions provided to the lab for this step is an important part of submitting samples for testing. For example, if there is a fluid pathway that should be flushed or filled, components that should be excluded, or if the sample should not be cut. These are just a few of the most common examples that we encounter as a testing laboratory or as regulatory consultants assisting with additional information requests during submission.
A topic for every device sample preparation is the extraction ratio that will be used. As an industry we reference ISO 10993-12:2021, specifically Table 1 (shown below).
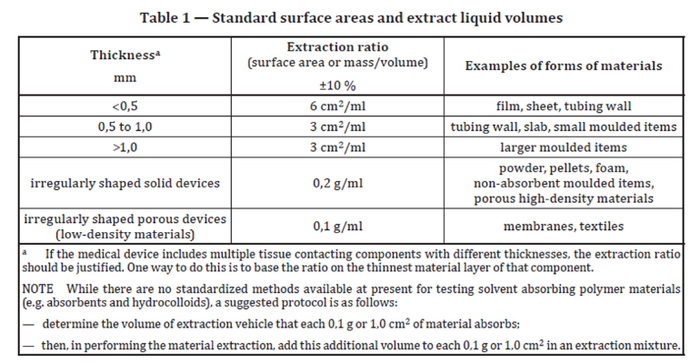
As each test performed has a minimum volume needed, this is an important initial consideration to determine if there is enough sample, where to apply the extraction fluid, and what components to include or exclude. When using the standards, it is important to understand and justify the approach used rather than randomly selecting parameters. To highlight, FDA’s guidance specifically asks for surface-area-to-volume ratios unless justification can be provided (section E). Fluid pathways that contact the patient should also be included in the extract (such as when the device has a lumen through which solutions are flushed into or onto the patient during surgery). For devices that only have indirect contact (such as syringes, external tubing intended for delivering fluid to patient, etc.), however, it is recognized that the fluid pathway typically has a very small internal surface area, much smaller than when the standard surface area ratio is applied. If the latter is used, it would lead to a much larger calculated fluid volume, which would just not fit inside the fluid path of the device. As such, the approach of “filled to capacity” has been considered as worst case for extract preparation and has often been accepted. However, it is always a good idea to demonstrate with simple math that this is indeed the case for your device.
Another important component of Test Article Preparation for Extract Testing is determining the time and temperature of the extract. Again, for the industry, we reference ISO 10993-12:2021, which allows for 37°C for 24 hours or 72 hours, 50°C for 72 hours, 70°C for 24 hours, or 121°C for 1 hour. The guidance document specifically reviews consideration for prolonged or long-term contacting devices where an extraction at 37°C may not be sufficient. This indicates that if the device has a limited (
Additionally, device indications and clinical-use scenarios may change over time. Thus, we recommend using 50°C whenever possible to avoid repeating testing. It is strongly advised that any changes to the most used extraction conditions be accompanied with a solid justification for why the altered parameters are better suited for testing and clinically representative; this will help to avoid unnecessary repetition of testing. For especially complex devices, it is also a good idea to prepare a Q-Sub or Pre-Sub application to run the chosen parameters and conditions by the agency before the testing is started.
Recording observations before and after extractions is not only listed in FDA’s guidance document, but it is also a requirement of ISO 10993-12:2021 section 11, which states, “The condition of the test extraction (color, clarity, presence of any particulates) shall also be described, and photographed if applicable).”
When issues arise with the condition of the extract, the lab should contact the sponsor to discuss and ask if the test will proceed or be discontinued. Regardless of the decision, investigation into the cause should follow. For example, if particulates are observed, they should be further analyzed to determine their source and if they would be expected clinically or if it is a residual of the sample preparation (such as cutting/bending of the sample).
An allowance is made in the Guinea Pig Maximization Test in ISO 10993-10:2021 for laboratories to perform positive control testing only every 6 months rather than for each testing day. FDA raises a concern that 6 months is too long to go between analysis of data. Thus, the recommendation is to ensure that the testing performed is within at least 3 months of the positive control testing. This is important information to consider, as this may require submission of further positive control data to the agency post testing to demonstrate proper control over the testing and animals.
There has been a lot of discussion in the industry to reduce in vivo testing, in which material mediated pyrogenicity (MMP) testing has been included. Currently, this is performed in rabbits, and there is not an alternate in vitro method available that is validated for medical devices. FDA does offer the option to review chemical characterization data (if available) for known material pyrogens. However, there is a caveat with this proposal. On nearly every chemical characterization (extractables and leachables) study (also often referred to as analytical chemistry testing), there are unknown compounds which are not able to be identified using the available libraries. And while there are certain thresholds available to assess unknowns, these thresholds are not applicable to pyrogens. Therefore, while the document lists this as an option, in practice it is not possible to address the pyrogenic potential of the device without an appropriate threshold.
As devices increase in complexity, assessing local tissue responses through implantation testing requires more initial consideration. There are standard implantation studies outlined in ISO 10993-6:2016 that indicate the species, sex, and number of animals as well as the number and size of implants to use. These studies are limited, as the recommended species is usually rabbits. While coupons can be manufactured to accommodate large devices and the small size of rabbits, in practice there is a limit to the number of materials that can be accurately analyzed; typically, no more than 4 materials may be included. A great alternative to the traditional implantation studies is to include implantation in a clinically relevant implantation study (functionality or performance testing). This requires that the laboratory performing the clinical testing review the ISO 10993-6 standard to ensure that the requirements from the standard are considered in the test protocol. Additionally, this protocol could include considerations for the assessment of additional toxicity endpoints (acute, subacute/subchronic, or chronic toxicity).
The aim of the FDA guidance document on the use of ISO 10993-1 is to provide clarity on what the agency expects for regulatory submissions. It gives pointers on risk assessments, testing to be performed, conditions for selecting and extracting devices for testing, and so on. The most significant change in the document is the inclusion of FDA’s position on intact skin-contacting materials. This is a welcome addition to ensure the reduction of animal testing, whenever appropriate. A review of the existing language in the document leads to our recommendation that the sample preparation be considered carefully and that a temperature of 50°C be used for extracts, where appropriate. More clarity in the expectations will enhance the ease of submission for both medical device manufacturers and for regulatory reviewers. We encourage making the submission process a dialogue, especially when submissions relate to complex devices, as this will lead to a better understanding of the concerns from the reviewer’s perspective and will help reviewers know how to best mitigate those concerns using the information at hand or with data to be generated.
Source & attribution: https://www.mddionline.com/regulatory-quality/fda-guidance-2023-on-iso-10993-1-what-changed-

In-vitro Diagnostic Medical Devices
North America: The FDA regulates in-vitro diagnostic...

The medical device industry has been significantly impacted by the Covid-19 pandemic, leading to ...
read more
The medical device industry is a dynamic and rapidly evolving sector, with advancements in techno...
read more
After watching this video, I came to understand the importance of having experienced individuals ...
read more
Let's talk about the often-overlooked but oh-so-important aspect of regulatory affairs:
read more
A fully integrated system for cybersecurity, design controls and risk management throughout the p...
read more
In case you missed it. 👇👇
A quick rundown of expert panel discussions, including MDR Article ...

Pictured is Tim Gooch presenting on the FDA's adoption of ISO 13485 at MD&M Minneapolis.
read more
The Dutch Health and Youth Care Inspectorate (IGJ) has conducted a survey of Netherlands-based ma...
read more
A service provider for medical device and related organizations looking for comprehensive regulat...
read moreWelcome to Prisma Compliance
These terms and conditions outline the rules and regulations for the use of Prisma Compliance Corp. 's Website, located at www.prismacompliance.com.
By accessing this website we assume you accept these terms and conditions. Do not continue to use Prisma Compliance if you do not agree to take all of the terms and conditions stated on this page.
The following terminology applies to these Terms and Conditions, Privacy Statement and Disclaimer Notice and all Agreements: "Client", "You" and "Your" refers to you, the person log on this website and compliant to the Company's terms and conditions. "The Company", "Ourselves", "We", "Our" and "Us", refers to our Company. "Party", "Parties", or "Us", refers to both the Client and ourselves. All terms refer to the offer, acceptance and consideration of payment necessary to undertake the process of our assistance to the Client in the most appropriate manner for the express purpose of meeting the Client's needs in respect of provision of the Company's stated services, in accordance with and subject to, prevailing law of us. Any use of the above terminology or other words in the singular, plural, capitalization and/or he/she or they, are taken as interchangeable and therefore as referring to the same.
Unless otherwise stated, Prisma Compliance Corp. and/or its licensors own the intellectual property rights for all material on Prisma Compliance. All intellectual property rights are reserved. You may access this from Prisma Compliance for your own personal use subjected to restrictions set in these terms and conditions.
You must not:
Republish material from Prisma Compliance
Sell, rent or sub-license material from Prisma Compliance
Reproduce, duplicate or copy material from Prisma Compliance
Redistribute content from Prisma Compliance
This Agreement shall begin on the date hereof. Our Terms and Conditions were created with the help of the Free Terms and Conditions Generator.
Parts of this website offer an opportunity for users to post and exchange opinions and information in certain areas of the website. Prisma Compliance Corp. does not filter, edit, publish or review Comments prior to their presence on the website. Comments do not reflect the views and opinions of Prisma Compliance Corp. ,its agents and/or affiliates. Comments reflect the views and opinions of the person who post their views and opinions. To the extent permitted by applicable laws, Prisma Compliance Corp. shall not be liable for the Comments or for any liability, damages or expenses caused and/or suffered as a result of any use of and/or posting of and/or appearance of the Comments on this website.
Prisma Compliance Corp. reserves the right to monitor all Comments and to remove any Comments which can be considered inappropriate, offensive or causes breach of these Terms and Conditions.
You warrant and represent that:
You are entitled to post the Comments on our website and have all necessary licenses and consents to do so;
The Comments do not invade any intellectual property right, including without limitation copyright, patent or trademark of any third party;
The Comments do not contain any defamatory, libelous, offensive, indecent or otherwise unlawful material which is an invasion of privacy
The Comments will not be used to solicit or promote business or custom or present commercial activities or unlawful activity.
You hereby grant Prisma Compliance Corp. a non-exclusive license to use, reproduce, edit and authorize others to use, reproduce and edit any of your Comments in any and all forms, formats or media.
The following organizations may link to our Website without prior written approval:
Government agencies;
Search engines;
News organizations;
Online directory distributors may link to our Website in the same manner as they hyperlink to the Websites of other listed businesses; and
System wide Accredited Businesses except soliciting non-profit organizations, charity shopping malls, and charity fundraising groups which may not hyperlink to our Website.
These organizations may link to our home page, to publications or to other Website information so long as the link: (a) is not in any way deceptive; (b) does not falsely imply sponsorship, endorsement or approval of the linking party and its products and/or services; and (c) fits within the context of the linking party's site.
We may consider and approve other link requests from the following types of organizations:
commonly-known consumer and/or business information sources;
dot.com community sites;
associations or other groups representing charities;
online directory distributors;
internet portals;
accounting, law and consulting firms; and
educational institutions and trade associations.
We will approve link requests from these organizations if we decide that: (a) the link would not make us look unfavorably to ourselves or to our accredited businesses; (b) the organization does not have any negative records with us; (c) the benefit to us from the visibility of the hyperlink compensates the absence of Prisma Compliance Corp. ; and (d) the link is in the context of general resource information.
These organizations may link to our home page so long as the link: (a) is not in any way deceptive; (b) does not falsely imply sponsorship, endorsement or approval of the linking party and its products or services; and (c) fits within the context of the linking party's site.
If you are one of the organizations listed in paragraph 2 above and are interested in linking to our website, you must inform us by sending an e-mail to Prisma Compliance Corp at info@prismacompliance.com. Please include your name, your organization name, contact information as well as the URL of your site, a list of any URLs from which you intend to link to our Website, and a list of the URLs on our site to which you would like to link. Wait 2-3 weeks for a response.
Approved organizations may hyperlink to our Website as follows:
By use of our corporate name; or
By use of the uniform resource locator being linked to; or
By use of any other description of our Website being linked to that makes sense within the context and format of content on the linking party's site.
No use of Prisma Compliance Corp. 's logo or other artwork will be allowed for linking absent a trademark license agreement.
Without prior approval and written permission, you may not create frames around our Webpages that alter in any way the visual presentation or appearance of our Website.
We shall not be hold responsible for any content that appears on your Website. You agree to protect and defend us against all claims that is rising on your Website. No link(s) should appear on any Website that may be interpreted as libelous, obscene or criminal, or which infringes, otherwise violates, or advocates the infringement or other violation of, any third party rights.
We reserve the right to request that you remove all links or any particular link to our Website. You approve to immediately remove all links to our Website upon request. We also reserve the right to amend these terms and conditions and its linking policy at any time. By continuously linking to our Website, you agree to be bound to and follow these linking terms and conditions.
If you find any link on our Website that is offensive for any reason, you are free to contact and inform us any moment. We will consider requests to remove links but we are not obligated to or so or to respond to you directly.
We do not ensure that the information on this website is correct, we do not warrant its completeness or accuracy; nor do we promise to ensure that the website remains available or that the material on the website is kept up to date.
To the maximum extent permitted by applicable law, we exclude all representations, warranties and conditions relating to our website and the use of this website. Nothing in this disclaimer will:
limit or exclude our or your liability for death or personal injury;
limit or exclude our or your liability for fraud or fraudulent misrepresentation;
limit any of our or your liabilities in any way that is not permitted under applicable law; or
exclude any of our or your liabilities that may not be excluded under applicable law.
The limitations and prohibitions of liability set in this Section and elsewhere in this disclaimer: (a) are subject to the preceding paragraph; and (b) govern all liabilities arising under the disclaimer, including liabilities arising in contract, in tort and for breach of statutory duty.
As long as the website and the information and services on the website are provided free of charge, we will not be liable for any loss or damage of any nature.
We employ the use of cookies. By accessing Prisma Compliance, you agreed to use cookies in agreement with the Prisma Compliance Corp. 's Privacy Policy.
Most interactive websites use cookies to let us retrieve the user's details for each visit. Cookies are used by our website to enable the functionality of certain areas to make it easier for people visiting our website. Some of our affiliate/advertising partners may also use cookies.
Necessary cookies are essential for the Website to function and cannot be disabled in our systems. These cookies are important since they allow you to browse our Website, they give you secure access to areas with personal information, they verify the authenticity of the visitor and they protect both the User and the Website from possible falsifications and misuse.
It is not mandatory to accept the use of these cookies. Statistical cookies help us to have a series of statistical data about the Website (visit or browsing data), in order to measure and improve the performance of our Website. Thanks to these cookies we can constantly improve your browsing experience.
This is the Cookie Policy for Prisma Compliance, accessible from www.prismacompliance.com
What Are Cookies
As is common practice with almost all professional websites this site uses cookies, which are tiny files that are downloaded to your computer, to improve your experience. This page describes what information they gather, how we use it and why we sometimes need to store these cookies. We will also share how you can prevent these cookies from being stored however this may downgrade or 'break' certain elements of the sites functionality.
How We Use Cookies
We use cookies for a variety of reasons detailed below. Unfortunately in most cases there are no industry standard options for disabling cookies without completely disabling the functionality and features they add to this site. It is recommended that you leave on all cookies if you are not sure whether you need them or not in case they are used to provide a service that you use.
Disabling Cookies
You can prevent the setting of cookies by adjusting the settings on your browser (see your browser Help for how to do this). Be aware that disabling cookies will affect the functionality of this and many other websites that you visit. Disabling cookies will usually result in also disabling certain functionality and features of the this site. Therefore it is recommended that you do not disable cookies. This Cookies Policy was created with the help of the Cookies Policy Generator.
The Cookies We Set
Account related cookies
If you create an account with us then we will use cookies for the management of the signup process and general administration. These cookies will usually be deleted when you log out however in some cases they may remain afterwards to remember your site preferences when logged out.
Login related cookies
We use cookies when you are logged in so that we can remember this fact. This prevents you from having to log in every single time you visit a new page. These cookies are typically removed or cleared when you log out to ensure that you can only access restricted features and areas when logged in.
Email newsletters related cookies
This site offers newsletter or email subscription services and cookies may be used to remember if you are already registered and whether to show certain notifications which might only be valid to subscribed/unsubscribed users.
Surveys related cookies
From time to time we offer user surveys and questionnaires to provide you with interesting insights, helpful tools, or to understand our user base more accurately. These surveys may use cookies to remember who has already taken part in a survey or to provide you with accurate results after you change pages.
Site preferences cookies
In order to provide you with a great experience on this site we provide the functionality to set your preferences for how this site runs when you use it. In order to remember your preferences we need to set cookies so that this information can be called whenever you interact with a page is affected by your preferences.
Third Party Cookies
In some special cases we also use cookies provided by trusted third parties. The following section details which third party cookies you might encounter through this site.
This site uses Google Analytics which is one of the most widespread and trusted analytics solution on the web for helping us to understand how you use the site and ways that we can improve your experience. These cookies may track things such as how long you spend on the site and the pages that you visit so we can continue to produce engaging content.
For more information on Google Analytics cookies, see the official Google Analytics page.
More Information
Hopefully that has clarified things for you and as was previously mentioned if there is something that you aren't sure whether you need or not it's usually safer to leave cookies enabled in case it does interact with one of the features you use on our site.
However if you are still looking for more information then you can contact us through one of our preferred contact methods:
Email: info@prismacompliance.com



